A WETTABILITY CAUSARIE
Bolting on Biogeochemistry to Explain the Wettability Enigmata
Until about the 1980’s, the industry generally accepted the fact that all formations must be water wet. The thinking was they were deposited in water and the oil simply migrated into the mobile pore space – unable to displace the immobile water. But, as well logs and lab procedures evolved, the industry began to encounter many field observations in oil entrapments that showed low initial water saturations (e.g., 10-20% Sow). It forced a change in thinking to recognize that some mixed wet (containing both immobile oil and water) reservoirs could occur. As a result, many theories were developed to try and explain the observed “mixed wettability” in rocks.
As more and more reservoir data rolled in, low Sow values were observed to be more prevalent in carbonate reservoirs. And, while folks have moved to an understanding that there are some mixed wet sandstones, they typically are not clean quartz sands – they usually have significant clay content or have a carbonate or sulfate-based cementing agents. The resins dropping out (i.e., highly polar hydrocarbons) to add a hydrocarbon layer on top of the water on the rock surface is probably the most common explanation for those mixed wet formations. Many researchers allude to that characteristic although some seem to believe the resins actually affix to the rock itself. In our work in trying to understand residual oil zones, we can now add another process (or dimension) which we very strongly believe is a large part of the story. The first piece relates to the natural anaerobic microbes that are indigenous in the subsurface. Their life processes involve moving electrons around. Two of their favorite atomic targets are sulfur and carbon. Each of those can change valence states with ease; for example, moving carbon in methane from having lost eight electrons in the process of inserting the carbon in calcite. Those “lost” electrons are utilized in converting the anhydrite to calcite and making a molecule of water and hydrogen sulfide. The carbon from the hydrocarbon source (shown here as methane) goes to the vacated lattice of the anhydrite molecule to make a new calcite lattice (CaSO4 goes to CaCO3). The sulfur atom combines with two of the hydrogen atoms to form H2S. The extra oxygen and two hydrogen atoms combine to form water. The figure below graphically displays this process.
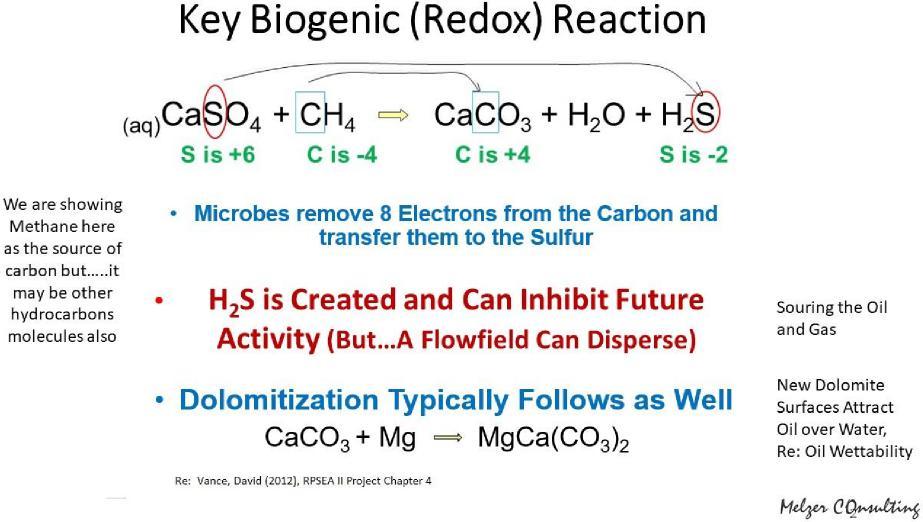
A recent presentation was recorded (see the video below) attempting to explain in more detail this process which leads to new dolomitic surfaces which, in turn, has a preference of attracting an oil molecule over water. This process does not occur unless the carbon, in the form of hydrocarbon, is present. And, in our case in the Permian Basin dolomites, lead to the souring of the oil and gas.
The significance of this in wettability space is that new crystal faces of calcite are formed which have a preferred affinity (polarity) to hydrocarbons over water. In the presence of hydrocarbons, and not just water, the new surfaces thereby create more immobile (residual) oil. As a sidebar discussion, the new calcite molecule can get exposed to Mg in the water and form dolomite – and, since crystalline dolomite is denser than limestone, porosity is created in these late stage diagenetic processes.
The industry has not spent much of their time to date in thinking about these processes for two reasons: 1) this mixed wettability realization thing is pretty new and 2) the reservoir researchers have been biased to observing original oil entrapments (main pay zones) that are static environments and do not have an associated flow field. This latter reason is really a key point. If the by-product H2S is emitted into a static condition, it will reach a state of water concentration (~200 ppm) that inhibits further activity by the microbes. So the oil wetting process is effectively limited by the buildup of the by-product H2S. In our work we looked at the laterally water-swept residual oil zones and their continuing flow-fields that allowed the microbial processes to progress essentially unabated. This process explained some other puzzles including 300’ thick residual oil zones with nearly constant Sor values which stood in direct defiance of the alternative explanations of transition zones with their Sor values declining rapidly with depth.
Once this model was in our heads, we then looked back at the main pay zones and thought about the original oil entrapping process. That entrapment phase created a flow field for a while and would seem likely to allow a bit of the mixed-wetting to occur. Once the invading hydrocarbons occupied all the displaceable pore space, the process ceased and microbes went inactive. Such would not be case where a flow field could remain as in a laterally displacing paleo entrapment (Type 3) ROZ where the by-product H2S could escape.
So how does all this relate to sandstones? If the reservoir and reservoir fluids have the necessary electron donor and receptor atoms, we can easily visualize mixed wet conditions. In some sandstones we know there are clays present. The Red Fork sandstone in Oklahoma is a case in point. As mentioned earlier, carbonate cements, clay particles or even sulfate waters can provide all the key ingredients to the exercise the process. Iron oxide cements can also contribute a microbial component. Lab work has also shown arkosic sandstones that are often mixed-wet. All of these could be factors explaining anomalously high Sor and low Sow values seen in those oil reservoirs. The parallel sidebar diagenesis discussion here may center around the formation of late stage formation of authigenic clays.
We also now believe that, in a few carbonates, we very likely have a nearly fully oil wet system – at least in so far as the contactable (non-occluded) pore space is concerned. The San Andres ROZs in West Texas fit this category beautifully. The main pay zones, conversely, fit the mixed wet category – likely due to the microbial self-limiting (MSL) presence of by-product H2S and their lack of a flow field.
Why Spend Time Worrying These Issues?
One can argue understanding these wettability processes and the degree to which reservoirs have been oil wetted have been key questions for estimating mobile plus residual oil targets for many decades. Obtaining samples from cores and cuttings has helped but actual quantification has been very problematic. Both the drilling fluid forces and depressuring of samples from in-situ conditions plays havoc with the true Sor and Sow estimates. Pressure core, sponge core, core vault and efforts to reconstruct in-situ conditions in the lab are all techniques that have attempted to side-step part of the sampling problems. The industry has made much progress but we often gloss over the uncertainties we are left with. They still remain in many formations. Finally, and as an example, let’s take a look at a mature water flood. In the regions where the water has swept the mobile oil, what is the residual oil saturation left after the water flood (Sorw)? That is the important question as it is precisely the target oil left for enhanced oil recovery. If the residual oil left behind is low, a follow-on EOR project will likely be unsuccessful. But if the water was inefficient in moving as much of the oil as in a high Sor reservoir condition, the EOR project will have plenty of target oil to be successful. Thus, identifying mixed- and fully oil-wet reservoirs becomes a key ingredient in the screening criteria for an EOR project. We believe adding in the reservoir diagenesis and microbial “bolt-on” thinking about wettability is thereby paramount.